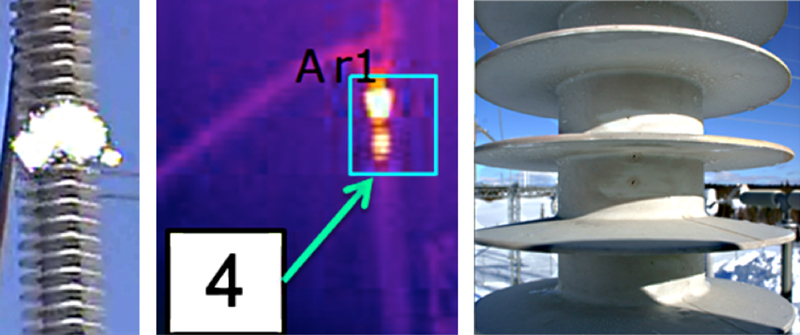
Several years ago, service inspections of composite station post insulators by utilities in Europe revealed instances of corona occurring not only close to the flange but also at some distance from the high voltage end. This finding was unexpected given that corona activity is usually linked to high electric field. Thermal inspection of these insulators detected hot spots in the same places where corona had been observed. Close visual inspection then revealed multiple punctures along the housing in those locations where corona and hot spots had been detected. Follow-up laboratory investigation found that the punctured areas were all marked by poor adhesion between core rod and polymeric housing. This suggested that one of the ways composite insulators can deteriorate and fail is from manufacturing processes that do not ensure proper adhesion along this critical interface.
This edited 2017 contribution to INMR by Dr. Andreas Bacher of Wacker Silicones discussed technologies that promote optimal adhesion between silicone elastomer and a substrate.
The different types of elastomers grouped in International Standard ISO 1629,4 are coded using a single letter, the most important of which are:
M: Rubbers having saturated carbon chain of the polymethylene type;
O: Rubbers having carbon and oxygen in the polymer chain; and
Q: Rubbers having silicon and oxygen in the polymer chain;
According to this standard, silicone elastomers are then defined by adding the substituent group on the polymer chain prior to the Q designation.thus leading to the following main groups:
MQ or polydimethylsiloxane (PDMS) that describes a polymer in which both side groups ‘R’ are methyl groups;
VMQ that describes a PDMS polymer in which small numbers of methyl groups have been substituted by vinyl groups;
PVMQ that stands for PDMS with small amount of methyl groups exchanged by phenyl groups; and
FVMQ that describes PDMS in which small numbers of methyl groups have been replaced by trifluoropropyl groups.
The silicone material used in medium and high voltage insulation applications belongs to VMQ silicone elastomers, which consist mainly of the silicone polymer described above along with reinforcing filler. Separation into different product groups among these elastomers is then made according to the different viscosities of the formulation as well as the cross-linking mechanism used. For example, 3 distinct groups are used in T&D applications: high consistency rubber (HCR), liquid silicone rubber (LSR) and room temperature vulcanizing (RTV) rubber. HCR products rely on both peroxide curing and platinum catalyst addition curing. Moreover, one can find addition cured LSR and RTV-2 formulations as well as condensation cured RTV-1 systems.
Adhesion Technologies
Adhesion of silicone elastomers to different substrates is an important topic for a wide range of applications. Ensuring optimal adhesion of silicone rubber housing to a core rod or tube substrate in composite insulators can be achieved using several processing methodologies.
• Application of primers;
• Surface treatment;
• Composite design with undercuts; and
• Use of self-adhesive grades of silicone rubber (not feasible for T&D applications).
Generating build-up of good adhesion between a substrate and a silicone elastomer using over-moulding requires a complex interaction of physical and chemical processes. Influencing factors include functional groups on the surface of the substrate and silicone, surface energy and operating temperature. For example, high surface energy with the presence of reactive chemical groups is necessary to achieve good bonding. Surface treatment with plasma, corona, Pyrosil® or flame treatment can generate these desired conditions but with relatively high investment in processing technology. Plasma, corona and flame treatment all generate reactive chemical groups on the surface of the substrate by applying energy with ionic plasma, corona discharge or natural gas flame respectively. By contrast, Pyrosil® technology is based on bringing new reactive groups to the surface by deposition of combustion chemical vapour (see Fig. 1).

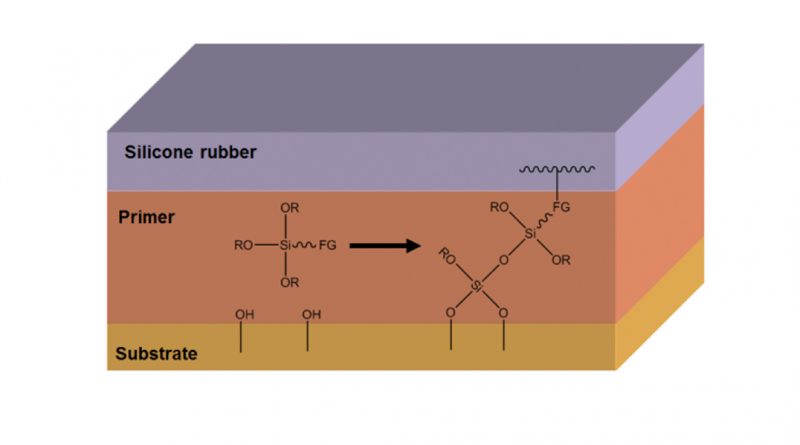
The gas flame is enriched with a precursor, an organo-silicon compound that is burned off during the process. The ash generated deposits as an amorphous silicate on the surface and this thin layer will form a strongly adhering coating that ensures good adhesion. Using a chemical primer can also establish chemical bonding between silicone rubber and substrate (see Fig. 2). All parameters necessary for the chemical reaction of the primer must be properly followed to ensure stable and reproducible results.
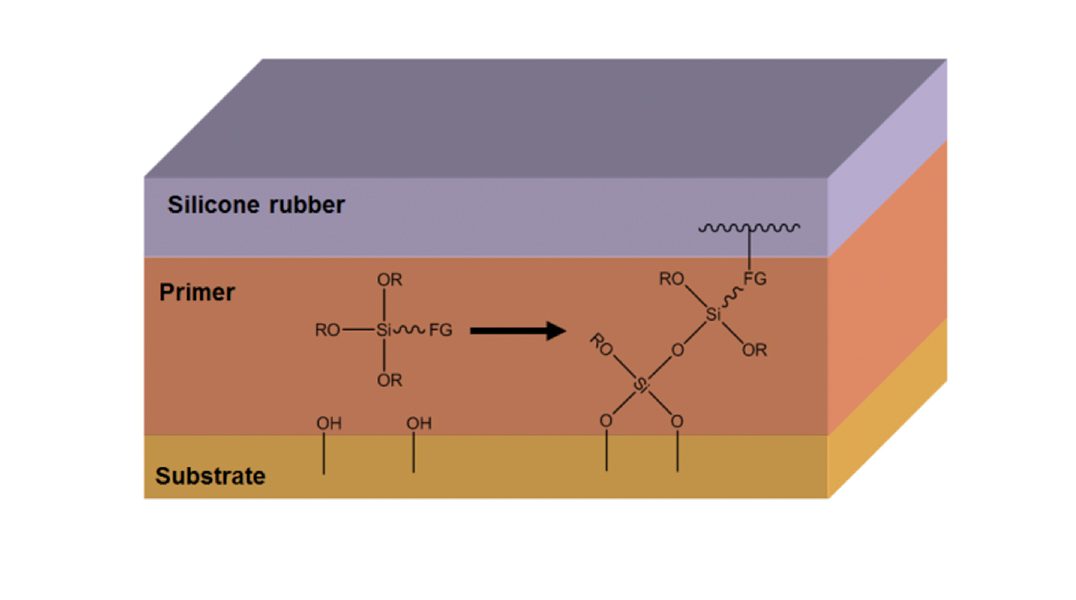
The primer layer is typically applied by means of spraying, brushing or dipping. After ‘flash-off’ of the solvent, the primer is cured with humidity from the ambient air. In some cases, baking the substrate with the cured primer layer improves adhesion. Finally, the silicone is cured onto the substrate by compression, transfer or injection moulding.
It is important to note that, apart from parameters such as humidity and temperature, other major factors are necessary to achieve a good result. For example, based on the material combination of substrate, primer and silicone rubber, adhesive force will decrease with storage time of the already primered part. Moreover, it is also important to know the surface conditions of the substrate. In general, a rough surface offers more surface area for establishing good chemical bonding. While it may seem common sense that applying more primer will improve effectiveness, such an approach is wrong. Rather, too thick a layer of primer levels out the surface roughness of the substrate and reduces contact surface. At the same time, build up of a thick layer of a resin results in a mechanically less stable, more brittle material that can break more easily (see Fig. 3).
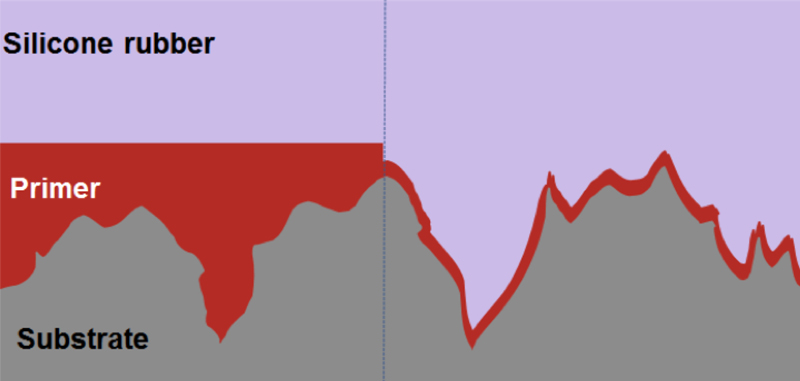
Appropriate preparation and cleaning of surfaces is also crucial for successful application of an adhesion promoter. Non-polar polymers or polluted surfaces can impede sufficient wetting of the entire surface and therefore reduce the adhesive surface.
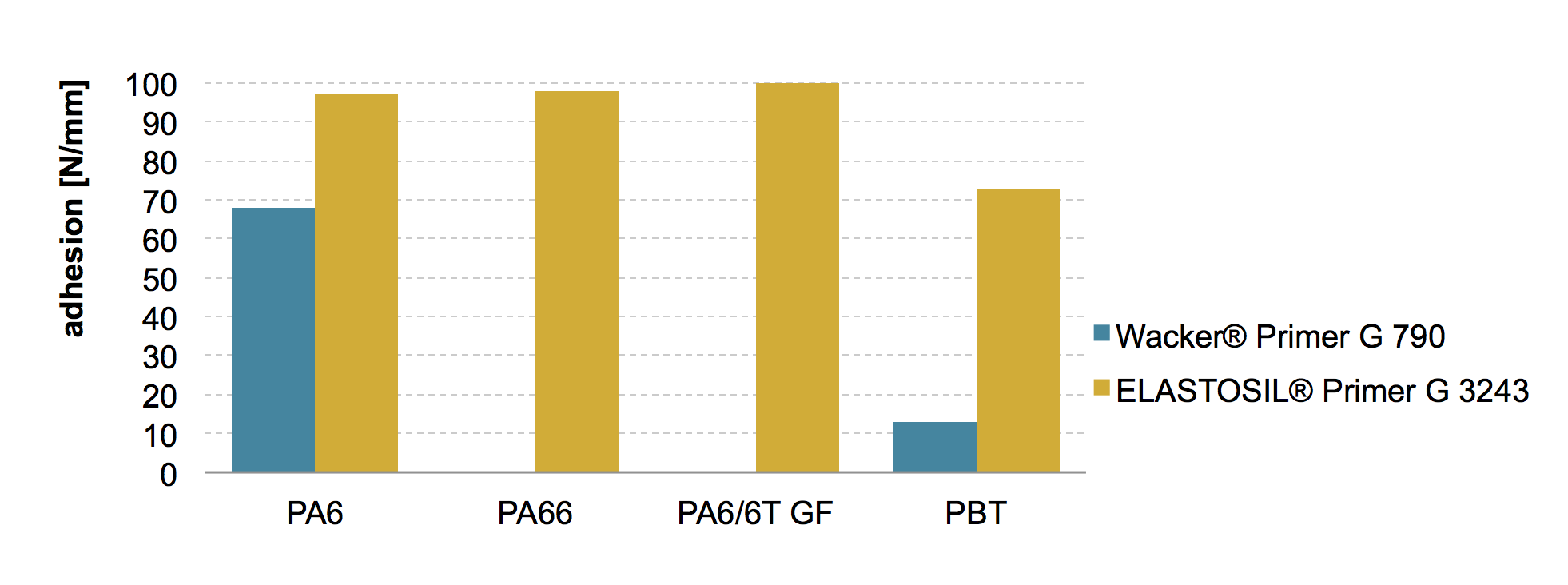
Adhesion primers can achieve good result in chemical bonding of silicone rubbers to various substrates if the processing complies with the optimal chemical process and parameters for the specific material combination under consideration. In order to accommodate this, different primers are available for the different cross-linking systems. These are designed to yield optimal adhesion through chemical bonding to the silicone in respect to its specific cross-linking system. For example, comparative trials using both addition curing and peroxide curing standard silicone with PA and PBT substrates, combined with a primer specifically designed for these curing systems, strongly confirmed this effect. Fig. 4 shows results using peroxide curing Elastosil® R 401/60 on substrates pre-treated with Wacker® Primer G 790 (recommended for addition curing products) and Primer Elastosil® G 3243 (recommended for peroxide curing formulations). It is obvious that the appropriate primer needs to be used to achieve optimal adhesion. Typical values reflecting a good adhesion force during these tests were above 10 N/mm.
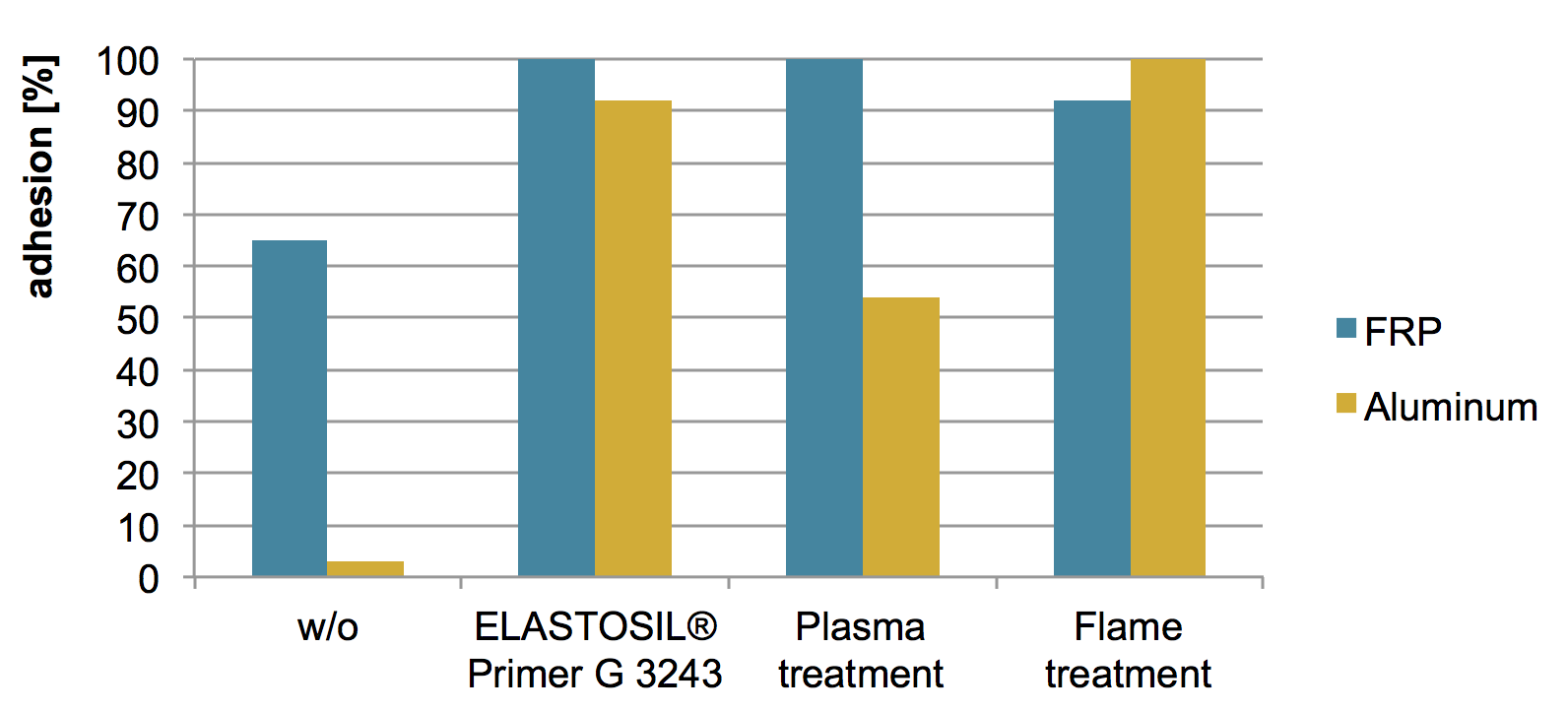
Comparison of different physical and the chemical pre-treatments of T&D relevant substrates such as FRP rods or tubes or aluminum was performed using the Powersil® 310 formulation. Since this is a peroxide curing formulation, Elastosil® Primer G 3243 was used. Fig. 5 clearly shows an increase in adhesion force using the pre-treated FRP compared to the untreated FRP. An even greater impact is evident in the case of aluminum.
It is evident from the above that a combination of different processes are available that can help achieve the best possible solution for any adhesion challenge involving silicone and a substrate, e.g. as in the case of composite insulators. Also, using the proper physical and chemical surface treatment will ensure good and stable adhesion.
References
[1] DIN ISO 1629, Rubber and lattices —Nomenclature, 2013.
[2] J. Lambrecht, Silicone Technology Review, INMR Issue 111, 2016
[3] ASTM D 1418, Standard Practice for Rubber and Rubber Latices —Nomenclature, 2015.
[4] Pachaly, B., Achenbach, F., Herzig, C., Mautner, K., Silicones, Winnacker/Küchler, Chemische Technik: Prozesse und Produkte, Band 5: Organische Zwischenverbindungen, Polymere, 2005.
[5] Tomanek, A., Silicones & Industry – a compendium for practical use, instruction and reference, Wacker Chemie AG, Carl Hanser Verlag, München, 1991.
[6] Sura Instruments GmbH, Das Pyrosil®-Verfahren, 2004.
[inline_ad_block]